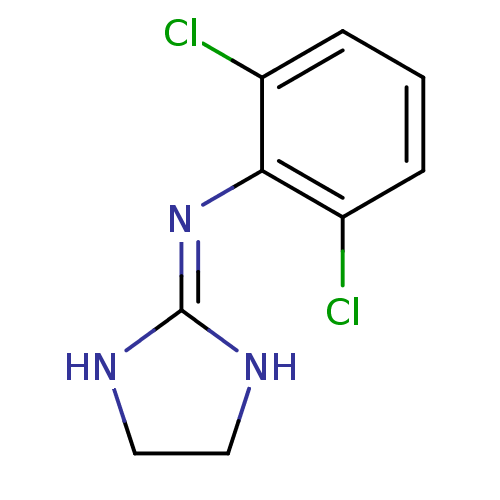
BDBM50016897 2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2)::2-(2,6-dichloroanilino)-2-imidazoline::CLONIDINE::N-(2,6-dichlorophenyl)-4,5-dihydro-1H-imidazol-2-amine::clonidine (amino form)
SMILES Clc1cccc(Cl)c1\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1
InChI Key InChIKey=GJSURZIOUXUGAL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50016897
Found 11 hits for monomerid = 50016897
Affinity DataKi: 6.20nMAssay Description:Binding affinity towards human Alpha-2B adrenergic receptor by the displacement of [3H]rauwolscineMore data for this Ligand-Target Pair
Affinity DataKi: 8.30nMAssay Description:Compound was tested in vitro for binding affinity against Alpha-2B adrenergic receptor from cloned rat RNG receptor transfected into Chinese hamster ...More data for this Ligand-Target Pair
Affinity DataKi: 8.30nMAssay Description:Binding affinity for rat Alpha-2B adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Displacement of [3H]RX821001 from human alpha2B adrenoceptor expressed in CHO cells after 60 mins by gamma counterMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:in vitro alpha-2B adrenergic receptor binding assay from rats ,using RX 821002 as the displaceable ligandMore data for this Ligand-Target Pair
Affinity DataEC50: 166nMAssay Description:Agonistic activity towards human Alpha-2B adrenergic receptor was measured as ability to inhibit forskolin-stimulated synthesis of cyclic adenosine m...More data for this Ligand-Target Pair
Affinity DataEC50: 1.18E+3nMAssay Description:Agonist activity at human alpha2B adrenoceptor expressed in CHO cells assessed as induction of extracellular acidification by microphysiometryMore data for this Ligand-Target Pair